Worldwide, approximately 5% of the population is currently affected by chronic inflammatory diseases, which have an underlying malfunction of the immune system in common. This group of diseases is very diverse. They include, among others, rheumatoid arthritis (RA), diabetes mellitus (type 1) and multiple sclerosis (MS). The interaction between genetic annex and external influences, such as the environment or lifestyle (e.g. nicotine), plays a major role in the development of the diseases. It leads to the immune system being less and less able to distinguish between “self” and “foreign” (self-tolerance). As a result, the body's own tissue structures are attacked by activated cells of the innate and adaptive immune system.
Currently, it is difficult to apply a timely and permanently effective pharmacotherapy to prevent irreparable damage to the target tissues without simultaneously impairing the immune protection of the affected individuals. Established synthetic or biopharmaceutical immunotherapeutic agents do not yet offer any chance of a cure and are associated with an increased risk of infection due to general immunosuppressive effects.
Targeting specific cells for improved efficacy and reduced risk of infection
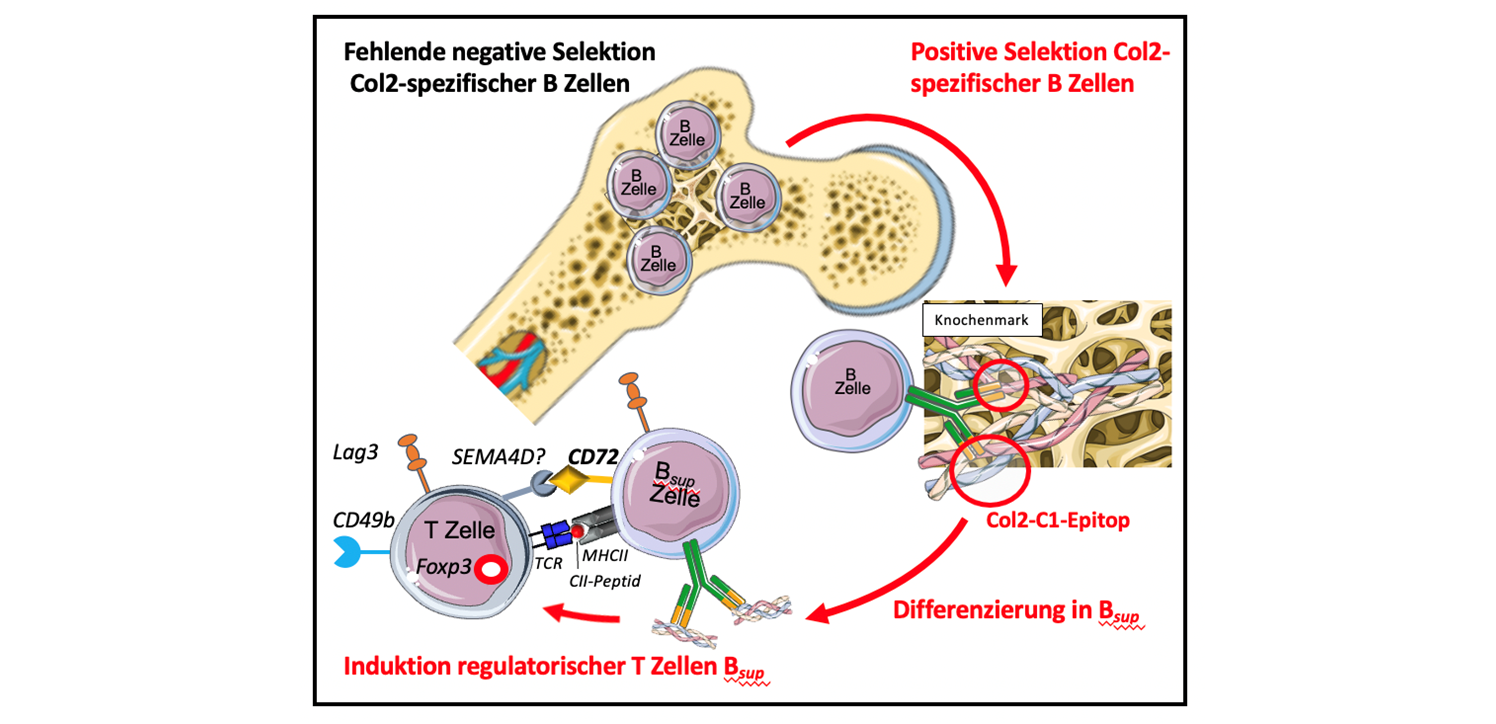
We have shown that in the mouse model, certain B cells, so-called collagen type II (Col2)-specific regulatory B cells, lead to the induction of regulatory T lymphocytes (Tregs). Tregs regulate the immune system and thus maintain self-tolerance and protect the body's own structures, in our case collagen structures. Based on these findings, we want to develop an antigen-specific B-cell-mediated tolerance-building strategy for the treatment of autoimmune diseases.
The demonstration of a “proof of concept” for the induction of regulatory T cells by antigen-specific B cells opens up new potential for the development of personalized therapeutic immunomodulation in the field of autoimmune diseases. The selective mode of action has the potential for improved efficacy with a reduced risk of infection, because no general immunosuppression occurs.
From pathogenesis research to therapeutic targeting – pooling expertise
To realize this project, we are drawing on expertise in pathogenesis research and established experimental disease models in the section of autoimmune diseases at the Fraunhofer Institute for Translational Medicine and Pharmacology in Frankfurt. We complement this expertise with cluster-specific expertise in the high-throughput sequencing of nucleic acids and bioinformatic analyses at the Fraunhofer Institutes for Interfacial Engineering and Biotechnology IGB in Stuttgart and for Toxicology and Experimental Medicine ITEM in Regensburg. The selection, synthesis and functionalization of aptamers as potentially selective ligands for the therapeutic targeting of lymphocyte subpopulations are carried out with the Fraunhofer Institute for Cell Therapy and Immunology IZI, Bioanalytics and Bioprocesses IZI-BB institute branch in Potsdam.
 Fraunhofer Cluster of Excellence Immune-Mediated Diseases
Fraunhofer Cluster of Excellence Immune-Mediated Diseases