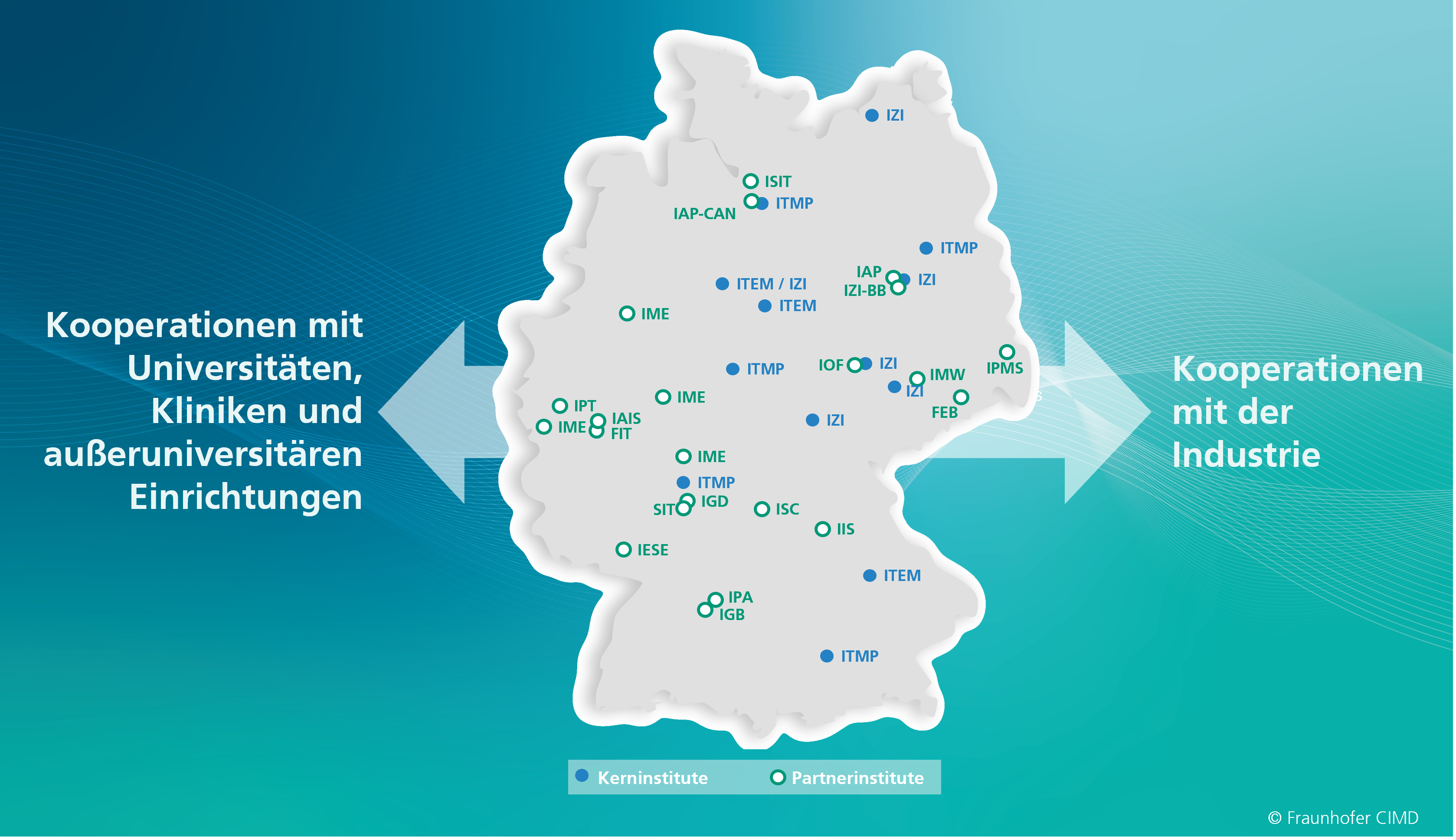
The Fraunhofer CIMD combines the interdisciplinary Fraunhofer expertise of three core institutes and approximately 14 partner institutes. In addition, the Fraunhofer CIMD has a large number of collaborations with universities, hospitals and non-university institutions, as well as with partners from industry.
We use the Fraunhofer-specific strengths in the field of medical translation and inter- and transdisciplinarity to gain scientific insights into the early detection, diagnosis and new therapeutic options for immune disorders and to optimize patient care.
 Fraunhofer Cluster of Excellence Immune-Mediated Diseases
Fraunhofer Cluster of Excellence Immune-Mediated Diseases